I told you that I would post more functional groups and I meant it. But I also want to make the ones I've already introduced clear. And condensed structures can confuse people. I heard you're easily confused. So we'll spend a bit more time getting acquainted with these. I think I covered the hydrocarbons well enough, so we'll focus on functional groups that contain heteroatoms.
More about alcoholsThe nature of the carbon that the hydroxyl group is attached to determines the type of alcohol here. First, there's a primary alcohol...

Note that the carbon attached to oxygen is attached to only one other carbon (in the R group). In a secondary alcohol, this carbon is attached to two other carbon atoms...

And as you might have guessed, in a tertiary alcohol, that carbon is bonded to three other carbons...
< img style="margin: 0px auto 10px; display: block; text-align: center; cursor: pointer; width: 320px; height: 240px;" src="http://1.bp.blogspot.com/_NvQHHJRdJ9o/SqTC252DPtI/AAAAAAAAAls/8wkQ_FWMAIA/s320/tertiary+alcohol.bmp" alt="" id="BLOGGER_PHOTO_ID_5378638103483465426" border="0" />If you were thinking that a quaternary alcohol would be one in which that carbon is attached to four other carbons, you sure are dumb. Carbon is tetravalent. You remember that, don't you? It can't form five bonds. There is no such thing as a quaternary alcohol.
If you were wondering, whether an alcohol is primary, secondary, or tertiary has important implications for its chemical properties, hence the distinction. This is also the case with amines, as I already alluded to, but in that case, it's how many carbons the nitrogen is attached to that determine which type of amine the molecule is. So there's some cool new information for you. But now for some clarity on material I alrea! dy covered in my last post.
Visualizing aldehydes & ketones
Here's the Lewis structure of an aldehyde...

And here, for contrast, is a ketone...

Notice the big difference: with an aldehyde, the oxygen is at the end of a chain and with a ketone, the oxygen is attached to a carbon that is somewhere in the middle of a chain. These structures both have a "carbonyl" group and their chemical properties are often similar, but they can be different in important ways and this distinction is certainly worth remembering.
Carboxylic acids and friends
Carboxylic acids get several other classes of compounds grouped with them as "derivatives of carboxylic acids" quite literally because carboxylic acids can be used to make these other compounds. I won't cover all of them because there's a whole chapter on this stuff and it's way later in my textbook. But because you're! slow, I worry about your ability to even deduce the general appearance of these groups from a condensed structure. So here's a carboxylic acid...
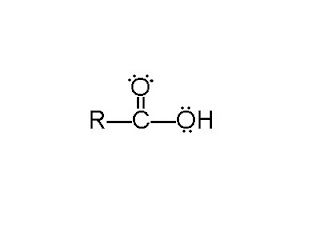
Like the aldehydes and ketones, there's a carbon double-bonded to an oxygen and single-bonded to an R-group. But the fourth bond isn't to hydrogen or another carbon. It's to oxygen, which itself is attached to hydrogen. Remember acidity? You know, that thing the last chapter was all about and such. And maybe you even remember that in my "Aspirin" post I said, of th! e carboxylic acid, "This arrangement of atoms makes it easy fo! r a cert ain reaction to occur. That reaction is a Brønsted-Lowry acid-base reaction." Really, it's not familiar. Whatever. That proton can totally come off.
Since I like functional groups so much, here are some more in condensed structure...
Acyl halideR—COX (like a carboxylic acid, but with the second oxygen replaced by a halogen)
Imine (imino group)R=N—R' (these come in multiple varieties and I haven't really studied them yet)
Peroxide (peroxy group)R—O—O—R' (the oxygens are actually attached to one another)
Nitrile (cyano group)R—C
≡N
Enough. We will now cover new functional groups as they come up.
do the following have a ketone functional group?








